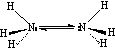Ammonia (NH3):
This is the only thermodynamically stable nitrogen hydride.
It is prepared by the Haber process:
Ammonia has a pyramidal structure, which undergoes rapid inversion (at a rate of 1010 s-1)
Ammonia is more soluble in water than any other gas. This is due to the formation of H-bonds between the NH3 and H2O molecules. It dissolves to give a basic solution. As well as being a Bronsted base (proton-acceptor), it is also a Lewis base (electron pair donor), and forms complexes with Transition Metal ions with its lone pair acting as a σ-donor.
Reactivity
Liquid ammonia is used as a non-aqueous solvent, although its self ionization is weaker than in water.Other properties
Hydrazine (N2H4):
Hydrazine has a 100% gauche structure at 25 oC, and is unstable with respect to formation of N2 and 2H2 This is a weaker base than ammonia, but it is dibasic.
It acts as a strong reducing agent, eg. in the Wolff-Kishner reduction, the driving force being the formation of the strong triple bond in N2.
Di-Imide (N2H2):
This is unstable and has the cis-structure.
Hydrazoic acid (HN3):
The azide anion (N3–) is linear (N–=N+=N–), symmetric and a stable leaving group.