The partial molar volume is broadly understood as the contribution that a component of a mixture makes to the overall volume of the solution. However, there is rather more to it than this:
When one mole of water is added to a large volume of water at 25ºC, the volume increases by 18cm3. The molar volume of pure water would thus be reported as 18cm3 mol-1. However, addition of one mole of water to a large volume of pure ethanol results in an increase in volume of only 14cm3. The reason that the increase is different is that the volume occupied by a given number of water molecules depends upon the identity of the surrounding molecules. The value 14cm3 is said to be the partial molar volume of water in ethanol.
In general, the partial molar volume of a substance X in a mixture is the change in volume per mole of X added to the mixture.
The partial molar volumes of the components of a mixture vary with the composition of the mixture, because the environment of the molecules in the mixture changes with the composition. It is the changing molecular environment (and the consequent alteration of the interactions between molecules) that results in the thermodynamic properties of a mixture changing as its composition is altered.
The partial molar volume, VJ, of any substance J at a general composition, is defined as:

where the subscript n’ indicates that the amount of all the other substances is held constant.
The partial molar is the slope of the plot of the total volume as the amount of J is changed with all other variables held constant:
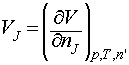
Once the partial molar volumes of the two components of a mixture at the composition and temperature of interest are known, the total volume of the mixture can be calculated from:Note that it is quite possible for the partial molar volume to be negative, as it would be at II in the above diagram. For example, the partial molar volume of magnesium sulphate in water is -1.4cm3 mol-1. i.e. addition of 1 mol MgSO4 to a large volume of water results in a decrease in volume of 1.4 cm3. (The contraction occurs because the salt breaks up the open structure of water as the ions become hydrated.)
![]()
The expression may be extended in an analogous fashion to mixtures with any number of components.
The most common method of measuring partial molar volumes is to measure the dependence of the volume of a solution upon its composition. The observed volume can then be fitted to a function of the composition (usually using a computer), and the slope of this function can be determined at any composition of interest by differentiation.