The Valence Shell Electron Pair Repulsion Theory is a way of predicting the shape of a molecule based on the number of bonding and lone pairs of electrons in a polyatomic species.
It is based on the fact that these electrons pairs interact and repel each other due the electrostatic repulsion. In doing so, they adopt a spatial arrangement such that they are as far apart as possible and the electrostatic repulsion is minimized.
The result of this is that the electron pairs around a given atom are arranged at the vertices of a regular polyhedron, with the number of vertices of the polyhedron being the number of electron pairs. These polyhedra are given in the table.
| Number of electron pairs | Spatial arrangement |
| 2 | Linear |
| 3 | Trigonal Planar |
| 4 | Tetrahedral |
| 5 | Trigonal Bipyramidal |
| 6 | Octahedral |
| 7 | Pentagonal Bipyramidal |
The arrangements of the electron pairs are therefore as shown below, but it should be noted that this is the arrangement of all electron pairs, both bonding and lone pairs. The shape of the molecule is, however, determined by the positions of the atoms, and not the electron pairs, and so the shape of the molecule may be different.
| Shapes of molecules for tetrahedrally arranged electron pairs | ||
| Molecule | Number of Lone pairs | Shape |
Methane: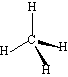 |
0 | Tetrahedral |
| Ammonia: |
1 | Trigonal Pyramidal |
| Water: |
2 | Bent |
The VSEPR can be extended to molecules with multiple bonds by noting that the multiple bond is treated the same way as a single bond. Hence, the sulphate ion, SO42-, which has two S=O bonds and two S-O bonds, is tetrahedral as the two types of bond are treated the same.
Modifications to the basic shape
The VSEPR model gives the basic arrangements of the electron pairs. However, it is important to distinguish between the lone and bonding pair electrons.
The electrons in a lone pair experience attraction to only one atom, as they are unshared, and so are considered to be closer to the nucleus than the electrons in a bonding pair. The degree of the repulsion between electron pairs therefore changes with the nature of the pairs being considered. It lies in the order:
Lone Pair – Lone Pair > Lone Pair – Bond Pair > Bond Pair – Bond Pair
This means that the presence of lone pairs will change the shape of a molecule. In the series above, methane has a H-C-H bond angle of 109.5o. In Ammonia, the greater repulsion from the lone pair means that the three bonding pairs are pushed closer together, and the H-N-H bond angle is 107o. In Water, there are two lone pairs, and hence the two remaining bonding pairs are pushed closer together still, and the H-O-H bond angle is 104o.
|
Structure prediction by VSEPR |
|
Lewis Structure: |
Shape: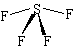 |
| The central S atom has 10 electrons around it (6 from the S and 1 from each of the 4 F atoms). These 10 electrons form 5 pairs, and so the basic structure is a trigonal bipyramid. The 5 pairs consist of 4 bonding pairs and 1 lone pair, and the lone pair occupies one of the trigonal positions, where repulsion is the lowest. The extra repulsion between the lone pair and the bonding pairs means that the axial bonds are pushed away from the lone pair, and the resulting shape is the see-saw shape shown. | |
| Other examples of structure prediction by VSEPR are shown in the structures of the interhalogen compounds. | |
Unpaired electrons: These are accounted for in structure prediction by VSEPR by treating an unpaired electron in the same way as a pair of electrons. The repulsion between an electron pair and a single electron is lower than that between electron pairs, and this will affect the shape in a similar way to the presence of the different repulsion between bonding and lone pairs.