Water is the most common solvent with respect to acids and bases. Often, the critical factor in determining the strength of an acid is played by the solvent, especially when that solvent is water.
Unfortunately, given that water is an ionising solvent, many organic acids do not dissolve sufficiently in it to begin with. However, if that hurdle is cleared, water is very effective as an ionising solvent, as it has a very high dielectric constant, and a high ion-solvating ability.
A high dielectric constant means that the force of attraction between any two oppositely charged ions will be lower than if they were in a solvent of low dielectric constant. Therefore, as the energy required to break them apart is less, they will be more stable in solution, and also less likely to recombine.
The ion-solvating ability helps, because ions in solution polarise their neighbouring solvent molecules, which stick to them in a semi-rigid frame, known as a solvent cage. Water is excellent at being polarised, and fairly small, and hence readily solvates anions and cations. e.g.:
 |
|
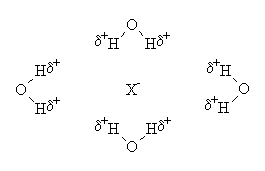 |
The actual number of solvating molecules depends on many factors, such as the size and charge of the ion, the size of the solvent molecules, their ease of polarisability, and their shape. The diagrams above are for illustrative purposes only.
One of the primary functions of the solvent, however, is that it should be able to function as a base (if an acid is in solution). The less basic the solvent, the less dissociated the acid.