We have already remarked that, for a pure substance, the chemical potential is just another name for the molar Gibbs energy. For a substance in a mixture, the chemical potential is defined as being the partial molar Gibbs energy:

i.e. the chemical potential is the slope of a plot of the Gibbs energy of the mixture against the amount of component J, with all other variables held constant:

The total Gibbs energy of a binary mixture is given by:In the above plot, the partial molar Gibbs energy is greater at I than at II.
![]()
(Compare the expression for the total volume of a binary solution in terms of its partial molar volumes given on the previous page.) The above expression may be generalised quite trivially to a mixture with an arbitrary number of components:
![]()
where the sum is across all the different substances present in the mixture, and the chemical potentials are those at the composition of the mixture.
This indicates that the chemical potential of a substance in a mixture is the contribution that substance makes to the total Gibbs energy of the mixture.
In general, the Gibbs energy depends upon the composition, pressure and temperature. Thus G may change when any of these variables alter, so for a system that has components A, B, etc, it is possible to rewrite the equation dG = Vdp – SdT (which is a general result that was derived here) as follows:
![]()
which is called the fundamental equation of chemical thermodynamics.
At constant temperature and pressure, the equation simplifies to:
![]()
Under these conditions, dG = dwn,max (as was demonstrated here), where the n indicates that the work is non-expansion work. Therefore, at constant temperature and pressure:
![]()
The idea that the changing composition of a system can do work should be familiar – this is what happens in an electrochemical cell, where the two halves of the chemical reaction are separated in space (at the two electrodes) and the changing composition results in the motion of electrons through a circuit, which can be used to do electrical work.
On a final note, it is possible to use the relationships between G and H, and G and U, to generate the following relations:
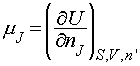
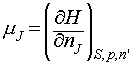
Note particularly the conditions (the variables that must be held constant) under which each relation applies.