Para: A position on a phenyl ring – see ortho.
Partial Pressure: The pressure that a gas which is a component of a mixture of gases would exert if there were no other gases in the container.
Particle in a box: In quantum mechanics, a common model in which a particle is confined to a region by potential energy curves that reach infinity. It is commonly, though not entirely accurately, used as a synonym for an infinite square well.
Pauling’s Rule: The pKa of a hydroxyacid may be predicted from the number of oxy- and hydroxy- groups it contains: for the acid E(O)m(OH)n, pKa = 8-5m.
Pauli: The Pauli principle states that in any atom, no two electrons may have the same four quantum numbers. Another way of stating this is to say that electrons in the same orbital must have opposite spins.
Penetration: The ability of an electron to get close to the nucleus and experience a high proportion of the nuclear charge.
Perfect Gas: (Ideal Gas) A gas which obeys exactly the relationship pV = nRT. Such a gas is a hypothetical model of a real gas in which the particles have zero size, and there are no interactions between particles except perfectly elastic collisions when they collide with each other.
Pericyclic Reactions: See the Pericyclics Section.
Periodic Table: The arrangement, by atomic number, of elements into groups with similar chemical properties.
Perpendicular: At 900 to something else.
Phase: A state of matter that is uniform throughout in both chemical composition and physical state.
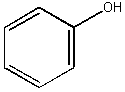
Phenol: C6H5OH
Photoelectron Spectroscopy: See Ultraviolet Photoelectron Spectroscopy (UV-PES).
Photoelectron: This is the electron emitted from a sample when it is irradiated with high energy ultraviolet radiation, as observed in Photoelectron Spectroscopy.
Photon: A discrete portion of an electromagnetic wave. The energy of a photon is given by E = hν, where ν is the frequency of the radiation.
Ph: Short for phenyl, denotes an aromatic ring (C6H5).
Pi bond: A bond between two atoms that consists of two electrons occupying a bonding molecular orbital that has a nodal plane in its wavefunction.
Plane-Polarized Light: Light in which the electric field of all of the photons is oscillating in the same plane.
Point Defect: This is a defect in a solid which is localized to a specific point in the lattice.
Point Group: The point group of an object is the group into which it is classified on the basis of the symmetry elements it possesses.
Polar Solvent: A solvent in which the molecules have a good solvating power by virtue of the fact that they have a large permanent electric dipole.
Polarisability: The ease with which the electron distribution of an atom or molecule can be distorted by an applied electric field.
Polarised: A bond is polarised if the electron density is not uniformly distributed along it, so that a dipole is created – this tends to happen when electronegative or electropositive (relative to carbon) elements are bonded to carbon. The bond will then have a partially positively charged end ‘δ+‘, and a partially negatively charged end ‘δ–‘.
Polydentate ligand: A ligand is polydentate if it may bond to the metal ion center in a complex through more than one atom, ie. if its denticity is greater than one.
Population of states: The distribution of a large number of systems amongst the energy levels available to them is represented by the population of states (the population of the different energy states available).
Precipitate: An insoluble compound which is the result of a reaction.
Primary: In the context of hydrocarbon chains and their functional groups, primary indicates that the carbon (or nitrogen) to which the functional group is attached has only one other carbon atom attached to itself (excluding any that may be in the functional group).
Products: The resultant molecules of a reaction are the products.
Promotion: The process whereby an electron is moved from an atomic or molecular orbital into one of higher energy.
Protonation: The instance of a substance gaining proton, i.e. being acidified.
Pr: Short for propyl, denotes a C3H7 group.
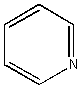
Pyridine: A ‘hetero-aromatic‘: C5H5N
p Orbital: An electron with one unit of angular momentum is contained in a p orbital.
pH: Defined as the negative log to base 10 of the hydrogen ion activity. A measure of the acidity of a solution – the lower the pH the more acidic the solution.
pKa: The prefix “p” merely denotes taking -log10 of whatever comes after the “p”. In this case, it means -log10Ka.
pKa: Defined as the negative log to base 10 of the acidity constant, Ka.