Crystal Field Theory describes the interactions between the ligands and the metal ion orbitals on an electrostatic basis, and examines the effect the ligands have on the metal center as a result.
However, the interaction between the ligands and the metal is generally stronger than simply an electrostatic interaction. There is generally some sharing of electrons between the orbitals, or the transfer of electrons from metal to ligand or ligand to metal, and so the interaction should properly be treated in terms of a bonding interaction.
The Ligand Field Theory builds up the interaction between metal and ligands in terms of covalent bonds between the ligand and metal. To do this, a molecular orbital (MO) diagram must be drawn.
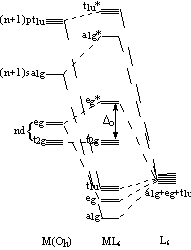
If we consider the octahedral arrangement of ligands, in order to draw the MO diagram we must first generate the symmetry adapted linear combination (SALC) of the ligand donor orbitals. If we consider only the s-orbitals on the ligands, then there are 6 orbitals and in an octahedral symmetry environment, they transform as a1g+eg+t1u. The metal orbitals to be considered are the nd-orbitals, and the (n+1)s- and (n+1)p-orbitals.
|
Molecular Orbital diagram for an metal-ligand complex in an octahedral field |
Whether one uses the Ligand Field or the Crystal Field description, the important result is that the metal d-orbitals become split in energy, by an amount known as the Ligand Field Splitting Parameter, Δo, in an octahedral field, which depends on both the identity of the metal and the ligands.Now, we see that the ligand field splitting arises from the fact that the metal d-orbitals of t2g symmetry are non-bonding, as there is no ligand SALC of the same symmetry with which they can interact, whereas the metal d-orbitals of eg symmetry become antibonding and so are raised in energy, because they interact with the ligand SALCs of eg symmetry.