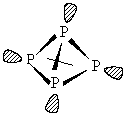
Phosphorus occurs in two forms: white phosphorus is made up of pyramidal P4 units, and is very reactive due to the bond strain in the cage (the P-P-P bond angle is 60o), and black phosphorus which is made up of extended layers of trigonal pyramidal coordinated P atoms. Structure of P4 in white phosphorus: The small bond angle in the tetrahedron means this is a highly strained, and therefore very reactive molecule.
Phosphorus forms binary compounds with virtually every metallic element, and many MP compounds have the ZnS (zinc blende) structure. However, compounds containing formally P3- are metallic and not ionic.
Phosphorus Hydrides
Phosphorus forms hydrides of the same stoichiometry as nitrogen, but the properties are very different.(see here for a comparison of nitrogen and phosphorus.)
Phosphine (PH3): This has the same pyramidal structure as ammonia, and burns in air after ignition. However, it is only sparingly soluble in water, unlike ammonia, and gives a solution which is neither basic nor acidic.
Diphosphine (P2H4): again, this has no basic properties, unlike hydrazine, N2H4.
Other Group 15 Hydrides
The stability of MH3 falls rapidly down the group, and SbH3 and BiH3 are very thermally unstable. This reflects the decreasing strength of the M-H bond strength down the group.
AsH3 and SbH3 are poisonous.