The aromatic ring is a very stable and electron rich entity – the delocalised π electrons lie above and below the plane of the ring and make it more attractive to electrophiles than nucleophiles. Substitution is likely to be the mechanism because this will return the aromaticity to the ring which is energetically favourable. However, because benzene itself is so stabilised it is also fairly unreactive – to the extent that it is often used as a solvent for organic reactions. Therefore highly reactive electrophiles are needed to react with it – some common reactions are given below;
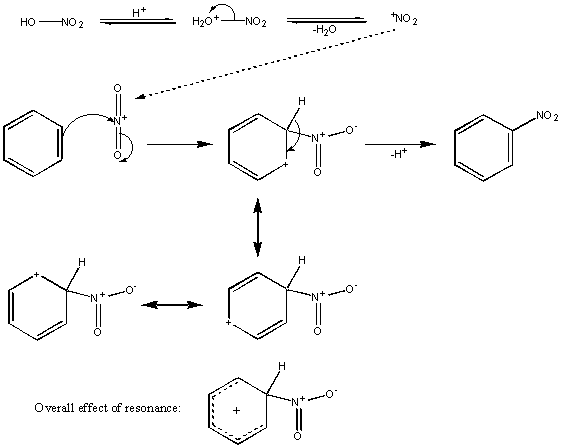
Nitration
The reagents for this are concentrated nitric and sulphuric acid – a so-called ‘nitrating mixture‘. The result of their combination is shown for clarity, but the important part of it is that the highly electrophilic species NO2+ (the nitronium ion) is created in situ;
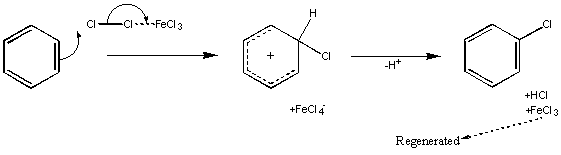
Halogenation
This utilises a Lewis Acid to activate the dihalogen molecule – examples of Lewis Acids used are AlCl3, FeBr3 and other combinations of metal and halide (the halide on the metal will be the same as the halogen used in the substitution).
Sulphonation
The active species here is SO3 – present in oleum, H2S2O7, made by dissolving SO3 in concentrated sulphuric acid;

Note how the sulphur atom in the centre of the SO3 is highly δ+ because of the three electron withdrawing oxygens around it – this makes it highly electrophilic. Also note that this reaction is reversible (as is the electrophilic substitution of I2 by mechanism 2).