Experimentally we find that the most probable speed increases as the temperature is increased, or as the moleclular mass is decreased.
We can say that in order to heat something, we either have to supply heat energy to it, or compress it (supplying work energy). In either case, according to the first law of thermodynamics (ΔU = q + w) we have increased the Internal Energy of the system. The Internal energy is the sum of the kinetic, potential, rotational, vibrational and electronic energies of all the components of the system, so when we increase the internal energy, we increase characteristics of the system that result in an increase of temperature.
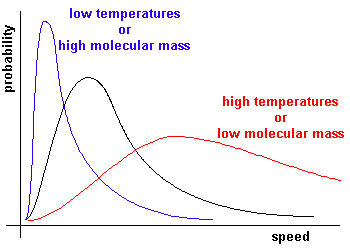
This corresponds to RT/M = (crms)2/3.