Metals have structures which may be discussed in terms of the close packing of spheres, and as a result have the high coordination numbers of the close packed systems. Ionic solids, however, have lower coordination numbers, and the discussion of the structure simply in terms of close packed species has to be adapted.
The idea of an ionic solid, though, depends on being able to define an ion. The Ionic Model treats a solid as being made up of oppositely charged spheres that interact by the coulombic forces between them, and the short range repulsive forces which occur between closed shell species at small separations.
The use of the idea of close packed spheres, and the holes within these structures is very useful in the discussion of the structures of ionic solids.
The structures of many ionic solids of the formula AB and AB2 may be visualized in terms of the close packed arrangement of the negatively charged anions, with the positively charged cations occupying the holes within the structure.
Characteristic structures of ionic solids
The simple structures come from the arrangement of the anions (though sometimes the cations) in the positions of the spheres in the fcc or hcp lattices, and the cations go into some or all of the octahedral and tetrahedral holes within the lattices.
Structures based on face centered cubic lattices
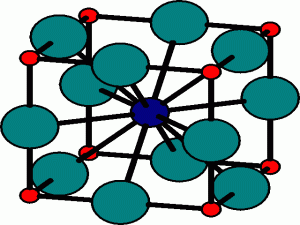
The Rock Salt structure
This the structure adopted by Sodium Chloride, NaCl. It is based on the fcc array of the large chloride anions, and the sodium cations occupy all the octahedral holes in the fcc lattice. However, it could also be seen as an fcc array of sodium ions, with the anions in all the octahedral holes.
Each ion is octahedrally coordinated by six counterions, and so this structure has so-called (6,6)-coordination, where the first number refers to the coordination of the cation and the second to the anion.
The structure beyond the first coordination sphere can also be visualized.
The Rock Salt, or NaCl, structure The sodium cations (green) occupy the octahedral holes in the fcc lattice of chloride anions (red). The extended coordination of the ions can be seen by considering the coordination of the sodium ion at the center of the unit cell. It has six nearest neighbours, which are the oppositely charged chloride anions, octahedrally arranged at the centers of the faces of the cube. The next nearest neighbours are 12 sodium cations, sited on the middle of each of the edges of the cube. Beyond that, there are 8 chloride anions situated at the corners of the cube.
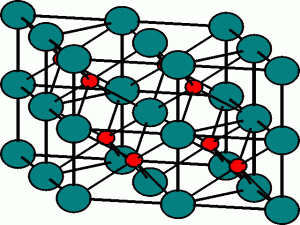
The Sphalerite structure
This is the structure adopted by Zinc Sulphide, ZnS, and is also known as the zinc blende structure.
Here there is an fcc array of sulphide anions, and the zinc cations occupy half the tetrahedral holes. There are two tetrahedral holes for each atom in the fcc array, and so the stoichiometry of the compound dictates that only half of them be occupied, so that there are the same number of cations as anions.
The cations are tetrahedrally coordinated by anions, and the anions are surrounded by eight tetrahedral sites, of which half are occupied, and hence the anions are also four-coordinate. The zinc blende structure therefore has (4,4)-coordination.
The Sphalerite, or Zinc Blende, structure The zinc cations (green) occupy half of the tetrahedral holes in the fcc lattice of sulphide anions (red). The Fluorite structure
This is the structure adopted by Calcium Fluoride, CaF2.
There is now an fcc array of calcium cations, and the fluoride anions occupy all of the tetrahedral holes. There are two tetrahedral holes for each atom in the fcc array, and so the stoichiometry of the compound dictates that both of the tetrahedral holes be occupied for each of the cationic fcc sites.
The anions are tetrahedrally coordinated by cations, and the cations are surrounded by eight tetrahedral sites, all of which are occupied, and hence the cations are eight-coordinate. The fluoride structure therefore has (8,4)-coordination.
The Fluorite, CaF2, structure
The fluoride anions (green) occupy all of the tetrahedral holes in the fcc lattice of calcium cations (red). The antifluorite structure is that adopted by compounds with the stoichiometry A2B, where A is the cation and B is the anion. Examples include potassium oxide, K2O. Here, the fcc array of oxide ions has potassium ions in all the tetrahedral holes, and there is a (4,8)-coordination.
Structures based on other cubic lattices
The Caesium Chloride structure
This is the structure adopted by Caesium Chloride, CsCl, and also by CsBr and CsI. It is formed when the anion and cation have similar sizes.
This is based on a simple cubic lattice of anions. In this the anions are not close packed, but the unit cell is a simple cube with an ion at the each of the corners. The cations are located at the center of the anionic cube.
Similarly, the structure can be considered as a simple cubic array of cations with the anions at the center of the cubes. It is fully understood as interleaved simple cubic lattices of cations and anions.
The Caesium Chloride Structure
The simple cubic array of chloride anions (red) has a cesium cation (green) at the center of the cube. There is an interleaved arrangement of simple cubic anionic and cationic arrays. The anions, at the corners of the cube, are coordinated to eight cations at the centers of each of the surrounding cubes, and the cations are surrounded by the eight anions at the corners of the cube. The cesium chloride structure therefore has (8,8)-coordination.
The Perovskite Structure
This is the structure adopted by Calcium Titanate, CaTiO3. It is the template for many compounds of the formula ABX3.
In this structure, there is a simple cubic array of B atoms (Ti), with the A atoms (Ca) occupying the center of the cube, as in CsCl, and the X atoms (O) being sited at the center of the 12 edges of the simple cube.
Therefore, the central A ion is coordinated by 12 X ions; the B ion is octahedrally coordinated by 6 X ions; and the X ion is linearly coordinated by 2 B ions.
The Perovskite Structure
The simple cubic array of titanium cations (red) has a calcium cation (blue) at the center of the cube, and oxide anions (green) at the center point of each of the edges of the cube of Ti ions. This is the prototype structure for compounds of the formula A2+B4+(O2-)3, as in perovskite itself, A3+B3+(O2-)3, and also in mixed oxides of formula A(B0.5B’0.5)O3, or A2BB’O6.
Structures based on hexagonal close packed lattices
The Wurtzite Structure
This is another structure adopted by Zinc Sulphide, ZnS, the difference from Zinc Blende being that the ions now occupy the sites in an hcp lattice.
Here there is an hcp array of sulphide anions, and the zinc cations occupy half the tetrahedral holes. There are two tetrahedral holes for each atom in the hcp array, and so the stoichiometry of the compound dictates that only half of them be occupied, so that there are the same number of cations as anions.
The Wurtzite, ZnS, Structure
The zinc cations (red) occupy half of the tetrahedral holes in the hcp lattice of sulphide anions (green). The cations are tetrahedrally coordinated by anions, and the anions are also tetrahedrally coordinated by cations. The Wurtzite structure therefore has (4,4)-coordination.
The local coordination of the ions at the next nearest neighbour level is the same in Wurtzite as Sphalerite, each ion being tetrahedrally coordinated by its counterions, but the coordination differs at the next nearest neighbour level.
The Nickel Arsenide Structure
This is the structure of NiAs, and is based on a distorted hcp array of Arsenide anions. By contrast with the wurtzite structure, however, which is also of formula AB, the cations now occupy all the octahedral sites rather than half the tetrahedral holes. There is one octahedral hole for each hcp lattice site, and so the AB stoichiometry is preserved.
The Nickel-Arsenide, NiAs, Structure
The hcp array of As anions (red) has Ni cations (green) in the octahedral holes. The local coordination of the anions and cations are different in this structure.
This structure is also adopted by NiS, FeS, and other sulphides. It is common where there are soft anions and soft cations, suggesting a degree of covalency.
The local coordination in the NiAs structure
In this structure, whilst the anions are based on an hcp lattice, they do not actually touch each other, and so the compound is not metallic.
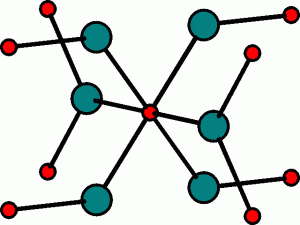
The Rutile Structure
This is the structure adopted by Titanium Oxide, TiO2.
Here, the anions occupy the hcp lattice, and the cations occupy half of the octahedral holes.
This results in the oxide ions being octahedrally arranged around the titanium cation, and the oxide ions themselves have titanium ions arranged in a planar triangle around them.
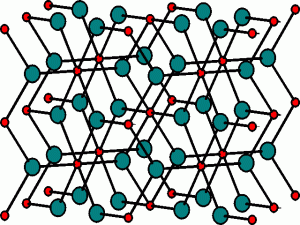
The Rutile, TiO2, Structure
The Rutile Unit Cell
The extended structure
The Ti cation (red) is octahedrally surrounded by O anions (green): the O anion has a planar triangle of Ti cations around it. The TiO6 octahedra stack vertically throughout the structure. The local arrangement of the anions and cations leads to the (6,3)-coordination in this structure.
The TiO6 octahedra are edge-sharing, and this results in chains of octahedra which run throughout the 3D-structure.