The halogens form a range of compounds with oxygen, but many of these are unstable.
Oxides are formed in the range E2O to E2O7.
Oxoacids are formed in the range HOE to HOEO3 (there is only HOF with fluorine).
Oxoanions are formed in the range EO– to EO4–.
Halogen oxides
Fluorine forms two compounds with oxygen. Oxygen difluoride, OF2, is formed from the reaction of F2 with hydroxide ions, and has the C2v structure of water as predicted by VSEPR. Dioxygen difluoride, FOOF, is produced by photolysis of a mixture of F2 and O2, and is a very good fluorinating agent, eg. in the fluorination of Plutonium.
| Preparation of OF2: | |
| Oxidation of Plutonium: |
Chlorine oxides occur with many chlorine oxidation numbers. Cl2O7 is the most stable of the oxychlorides. Cl2O has the bent structure of F2O, but here the Cl-O-Cl bond angle is smaller due to the presence of Opπ-Cldπ interactions, which favour the use of p rather than sp3-hybrid orbitals on the O for bonding, and hence have a bond angle closer to the 90o of the orthogonal p-orbitals.
| OS(Cl) = +1 | OS(Cl) = +4 | OS(Cl) = +6 | OS(Cl) = +7 |
| Cl2O, a brown-yellow gas | ClO2, a yellow gas | Cl2O6, a dark red liquid | Cl2O7, a colourless liquid |
ClO2 is used as a bleaching agent and disinfectant when used in dilute solutions. The oxidizing properties of ClIV make it useful for these purposes.
Halogen Oxoacids and Oxoanions
The structure of the oxoacids of the halogens can be used to predict their acidities, using Pauling’s rule, ie. pKa = 8-5p for the acid (O)pE(OH)q. The usefulness of this relationship can be seen in the oxoacids of chlorine.
| HOCl | hypochlorite | p = 0:8-5p = 8 | pKa = 7.53 | |
| HOClO | chlorite | p = 1:8-5p = 3 | pKa = 2.00 | |
| HOClO2 | chlorate |  |
p = 2:8-5p = -2 | pKa = -1.2 |
| HOClO3 | perchlorate |  |
p = 3:8-5p = -7 | pKa = -10 |
The pKa of HIO6 is only 3.29, and this seems high, but if one looks at the structure, we see that it is (HO)5IO, and so p=1 and the pKa is predicted to be 3. The rule is therefore fairly accurate, and can also be used as a tool for predicting the structure.
It can be seen that HClO4 is a very strong acid, and so the conjugate base, ClO4–, is a very weak base. It is also a very poor Lewis base, and does not form complexes. However, it is also a strong oxidizing agent, and solid perchlorate compounds can form metastable complexes which are explosive.
![]()
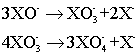
The oxidizing power of the halogen oxoanions leads to the instability of HOF. Above -40oC, it reacts with water to give HF and hydrogen peroxide. The XO– ion is also prone to disproportionation.