Organic acids have a wide range of strengths. The following discussion will explain some of the trends observed, by reference to Brønsted acids.
For example, consider the difference between ethanol and ethanoic acid. The two molecules both have an acidic proton (H+) attached to a heteroatom (in this case, oxygen):
Deprotonation of ethanol:
Deprotonation of ethanoic acid:
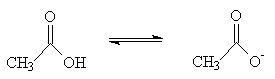 |
So why does ethanoic acid have a pKa = 4.8, and ethanol have a pKa = 15.9? Remembering that pKas are on a logarithmic scale, this means that ethanoic acid is 100,000,000,000 times stronger than ethanol.
There are two factors involved here. Firstly, the carbonyl (C=O) group of ethanoic acid is electron withdrawing with respect to the CH2 group of ethanol. Thus, the negative charge on the conjugate base of ethanoic acid is better accommodated.
The most important factor, however, is resonance stabilisation:
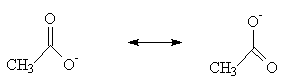 |
Neither of the two representations of the ethanoate ion, shown above, is strictly correct. The two forms are of equal energy, but the “real” picture of the ion would show that the negative charge is spread over the the CO2– section. This lowers the energy of the ion, and hence favours the dissociated form.
According to the scheme:
The previous discussion was an example of stabilisation of A–, thus moving the position of equilibrium to the right. However, the stability of HA will also have an effect on the position of equilibrium, i.e. the more stable HA is with respect to A–, the less strong an acid it will be.