A rearrangement is an intramolecular reaction – i.e. a single molecule reacting with no external input. The topic of rearrangements is extremely nebulous and hence very difficult to cover systematically. Therefore the best approach is to look through the following few pages of (mostly) well-known rearrangements and try to deduce your own patterns and similarities.
The theme uniting the reactions on this page is that they all involve an electron deficient nitrogen atom which receives electron density in the course of the rearrangement.
Sommelet-Hauser
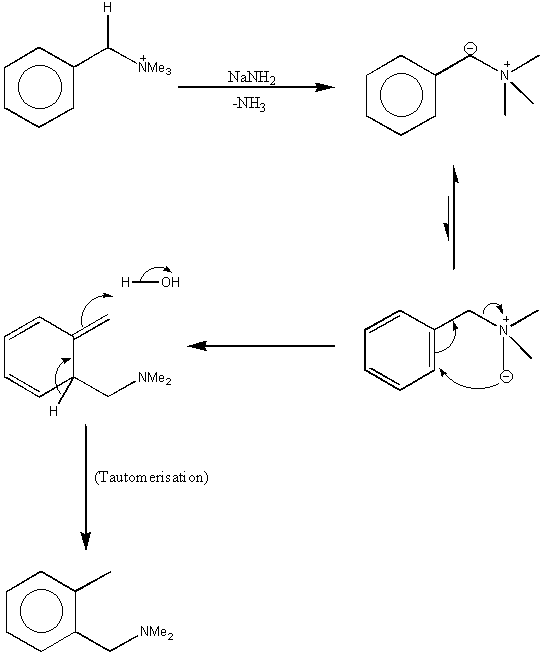 |
Points to note:
i. In the first step, the –NH2 takes the most acidic proton – where the resultant anion can be stabilised both by the N+ and by the phenyl ring (see here for the resonance stabilisation of benzylic anions). ii.From this anion no rearrangement can occur – however, step 2 shows a small equilibrium that is present and as the anion on the right goes through with the rearrangement, the eqm is pulled through until all the compound has rearranged. iii. The tautomerism labelled in step 4 will occur because the ring is returned its aromaticity – energetically favourable. |
Beckmann
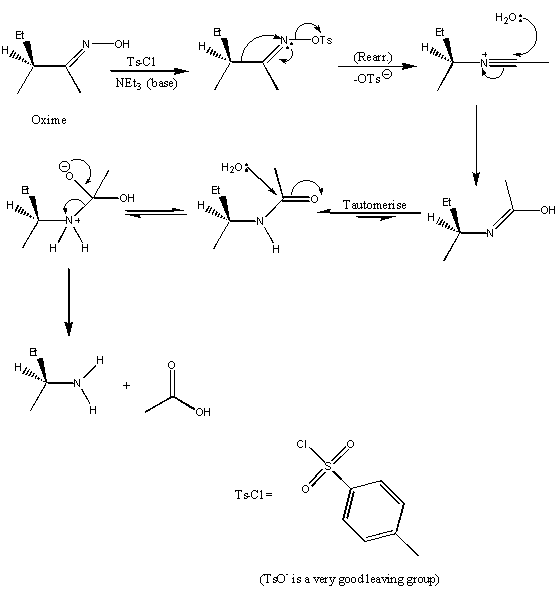 |
Points to note:
i. The actual rearrangement step is labelled – it is important to note that it is always the group trans to the oxygen which migrates. ii. After the rearrangement a simple amide hydrolysis follows. iii. Note also that the chiral group on the oxime retains its configuration throughout the rearrangement. |
Hofmann
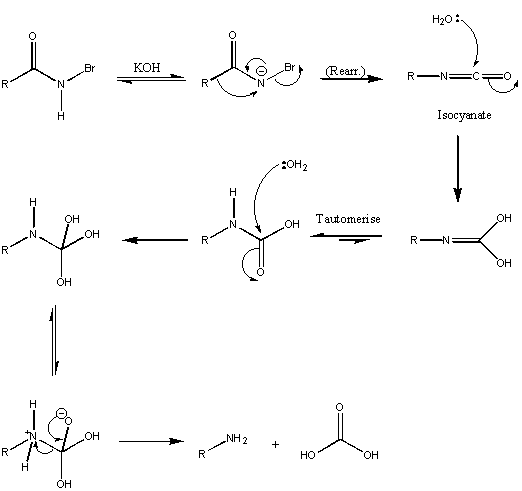 |
The isocyanate is an intermediate in the reaction and readily hydrolyses through to amine plus acid.